Radionuclides in the Ocean
Sea water is slightly radioactive: it contains a small but significant amount of radioactive elements that undergo spontaneous radioactive decay and produce energy, subatomic particles, and a remainder, or daughter nucleus, smaller than the original. The particles include alpha particles (two neutrons plus two protons), beta particles (electrons), and gamma energy. The radioactive elements are called radioactive isotopes, or radionuclides, or nuclides. Nearly all of the radioactive material in the ocean is natural, and represents material that has been on Earth since its formation.
What is Radioactivity?
Table 1 on page 22 summarizes some of the radioactive nuclides found in the ocean. Each one of these substances will undergo a spontaneous decay at a well-known rate as described by its half-life. That means, essentially, that its nucleus will explode. The half-life represents the amount of time that must pass for half of a group of atoms to undergo decay. Radioactivity is often expressed as decays per second, a unit referred to as a Becquerel (Bq). It is also sometimes expressed in Curies (Ci), which is the activity (corrected concentration) of a gram of 226 Ra, named after Marie Curie. A Curie equals 3.7 × 10 10 Bq.
The first question most people ask about radioactivity in the natural environment concerns health risks. The following list shows the radioactivity found in a typical adult human body of 70,000 grams (about 154 pounds). (A milligram (mg) is 10 −3 gram; a microgram (μg) is 10 −6 gram; a picogram (pg) is 10 −12 gram.)
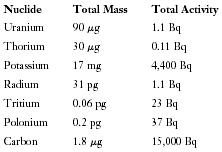
| Nuclide | Total Mass | Total Activity |
| Uranium | 90μg | 1.1 Bq |
| Thorium | 30μg | 0.11 Bq |
| Potassium | 17 mg | 4,400 Bq |
| Radium | 31 pg | 1.1 Bq |
| Tritium | 0.06 pg | 23 Bq |
| Polonium | 0.2 pg | 37 Bq |
| Carbon | 1.8μg | 15,000 Bq |
All people carry their own burden of radioactivity, mostly consisting of 40 K and 14 C. Exposure to additional amounts, especially large amounts at close range over a long period of time, can cause radiation sickness and even death. However, this does not result from exposure to normal sea water.
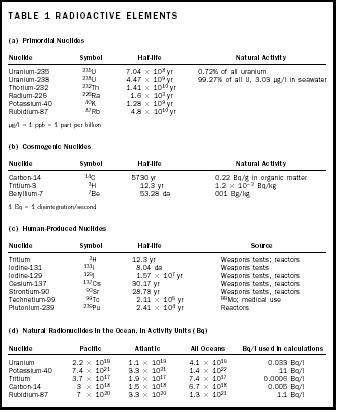
| TABLE 1 RADIOACTIVE ELEMENTS | ||||
| (a) Primordial Nuclides | ||||
| Nuclide | Symbol | Half-life | Natural Activity | |
| Uranium-235 | 235 U | 7.04 × 10 8 yr | 0.72% of all uranium | |
| Uranium-238 | 238 U | 4.47 × 10 9 yr | 99.27% of all U, 3.03 μg/l in seawater | |
| Thorium-232 | 232 Th | 1.41 × 10 10 yr | ||
| Radium-226 | 226 Ra | 1.6 × 10 3 yr | ||
| Potassium-40 | 40 K | 1.28 × 10 9 yr | ||
| Rubidium-87 | 87 Rb | 4.8 × 10 10 yr | ||
| μg/l = 1 ppb = 1 part per billion | ||||
| (b) Cosmogenic Nuclides | ||||
| Nuclide | Symbol | Half-life | Natural Activity | |
| Carbon-14 | 14 C | 5730. yr | 0.22 Bq/g in organic matter | |
| Tritium-3 | 3 H | 12.3 yr | 1.2 × 10 −3 Bq/kg | |
| Beryllium-7 | 7 Be | 53.28 da | 001 Bg/kg | |
| 1 Bq = 1 disintegration/second | ||||
| (c) Human-Produced Nuclides | ||||
| Nuclide | Symbol | Half-life | Source | |
| Tritium | 3 H | 12.3 yr | Weapons tests; reactors | |
| Iodine-131 | 131 I | 8.04 da | Weapons tests | |
| Iodine-129 | 129 I | 1.57 × 10 7 yr | Weapons tests, reactors | |
| Cesium-137 | 137 Cs | 30.17 yr | Weapons tests, reactors | |
| Strontium-90 | 90 Sr | 28.78 yr | Weapons tests, reactors | |
| Technetium-99 | 99 Tc | 2.11 × 10 5 yr | 99 Mo; medical use | |
| Plutonium-239 | 239 Pu | 2.41 × 10 4 yr | Reactors | |
| (d) Natural Radionuclides in the Ocean, in Activity Units (Bq) | ||||
| Nuclide | Pacific | Atlantic | All Oceans | Bq/l used in calculations |
| Uranium | 2.2 × 10 19 | 1.1 × 10 19 | 4.1 × 10 19 | 0.033 Bq/l |
| Potassium-40 | 7.4 × 10 21 | 3.3 × 10 21 | 1.4 × 10 22 | 11 Bq/l |
| Tritium | 3.7 × 10 17 | 1.9 × 10 17 | 7.4 × 10 17 | 0.0006 Bq/l |
| Carbon-14 | 3 × 10 18 | 1.5 × 10 18 | 6.7 × 10 18 | 0.005 Bq/l |
| Rubidium-87 | 7 × 10 20 | 3.3 × 10 20 | 1.3 × 10 21 | 1.1 Bq/l |
Types of Radioactive Nuclides on Earth.
Three types of radioactive nuclides exist on Earth: primordial nuclides, cosmogenic nuclides, and humanmade radionuclides.
- Primordial Nuclides. In the ocean, these include 40 K, 87 Rb, 238 U, 235 U, and 232 Th. All have extremely long half-lives and wide distribution throughout Earth and the ocean.
- Cosmogenic Nuclides. These are created by bombardment of upper atmosphere gases by cosmic radiation from space. Especially important among the cosmogenic nuclides are 14 C, 7 Be, and 3 H (tritium). Because they originate from the atmosphere, they enter the ocean from its surface.
- Human-made Radionuclides. Human beings have been concentrating, creating, and using radionuclides for a century, and have added to the inventory of natural radionuclides, although the amount added is small compared to natural amounts. Among the important artificial nuclides are tritium, ( 3 H), 131 I, 129 I, 137 Cs, 90 Sr, 99 Tc, and 239 Pu. Weapons testing and waste from nuclear reactors are two of the main sources from which these nuclides may enter the ocean, in both cases through its surface waters. In addition, disposal of nuclear waste such as submarine reactors in the ocean has created point sources of the material on the sea floor.
The primordial radionuclides include the three radioactive decay series as defined by their longest lived parent, 238 U, 232 Th and 235 U, respectively. Each of these nuclides decays to form a series of daughters that are radioactive in turn; each series finally terminates in a stable (non-radioactive) Pb (lead) nuclide. These decay series (shown below) represent a set of isotope tracers that helps illustrate how the ocean works as a system.

| TABLE 2 RADIOACTIVE DECAY CHAINS | ||||||||||||
| (a) Uranium-238 (99.3% of all U) | ||||||||||||
| 238 U | 234 Th | 234 U | 230 Th | |||||||||
| (4.47 × 10 9 y) | (24.1d) | (2.45 × 10 5 y) | (7.5 × 10 4 y) | 226 Ra | 222 Rn | 210 Pb | 210 Po | 206 Pb | ||||
| (1.6 × 10 3 y) | (3.82d) | (22.3y) | (138.4d) | (stable) | ||||||||
| (b) Uranium-235 (0.7% of all U) | ||||||||||||
| 235 U | 231 Th | 231 Pa | 227 Ac | 207 Pb | ||||||||
| (7.04 × 10 8 y) (25.5hr) | (3.28 × 10 4 y) (21.8y) | (stable) | ||||||||||
| (c) Thorium-232 | ||||||||||||
| 232 Th | 228 Ra | 228 Th | 224 Ra | 208 Pb | ||||||||
| (1.41 × 10 10 y) (5.76y) | (1.91y) | (3.66d) | (stable) | |||||||||
| Arrows indicate decay from parent to daughter; less important nuclides have been left out in case. Half-lives are given under each isotope. | ||||||||||||
| y = year d = day hr = hour |
Radioactivity in the Ocean
The partial inventory of natural radionuclides in sea water in Table 1 (see page 22) amounts to 1–2 × 10 22 Bq, without including the uranium daughters or the 232 Th series nuclides. Human-made nuclides in the ocean have been estimated to be 85 × 10 15 Bq directly dumped, 1.5 × 10 18 Bq from fallout, and 1 × 10 17 Bq from reprocessing plant effluent. The natural radionuclides are greater in abundance. The distribution of the artificial nuclides is very localized in some cases, which causes obvious concerns about health consequences.
Radionuclides in the ocean can sometimes be used as tracers of natural processes, and the half-lives or rates of disappearance behave like built-in clocks to help scientists determine the rate at which those processes take place. Notice in the decay schemes of these series (see Table 2 on page 23) that the daughters include nuclides that represent different chemical elements, and therefore different chemical behaviors, and have a whole range of different half-lives. It is important to keep track of the chemical behavior of each of these elements in the environment.
For example, U is quite soluble in sea water, Th is nearly totally insoluble, Ra is soluble, and Rn is a noble gas that is soluble in water and can escape into the atmosphere. As long as all the members of the 238 U decay series are kept in a closed container, they will eventually reach the state where they all are decaying with the same number of decays per second, because the half-life of the first decay is so long relative to subsequent decays. This state is called secular equilibrium, and will continue indefinitely unless some of the material escapes the container, or extra material is added.
In the environment however, the different nuclides can readily be separated because of their chemical differences. An activity ratio between parent and daughter either greater than or less than 1 shows that separation has taken place. For example, the depth profiles below show the amounts of 238 U, and its daughter 234 Th, in particles and water sampled from the eastern Pacific Ocean. All the samples show ratios lower than 1, which means that part of the 234 Th has attached to particles that have been removed from the area. Above the thermocline at 100 meters (328 feet) exists a layer of particle-rich water. The particles in this layer show an increase in the 234 Th-to- 238 U ratio that is mirrored by a descrease in the ratio for dissolved material. These particles contain adsorbed 234 Th that is being removed and transported out of the area by particle settling.
Depth (m) 1020300
100
200
300
Temperature (˚C)
Nitrate (μM)
102030
Pigments (mg m −3 )
0.10.30.50.7
234 Th/ 238 U Activity Ratio
0.20.61.0
part. total diss.
The long-lived 238 U parent is soluble in sea water, and it remains at a constant concentration over depth. The 234 Th daughter has a short half-life, about 24 days, and is very likely to stick to any particle near it, including the organic debris involved in biogeochemical cycling in the ocean. For that reason, it is lower in activity than its parent 238 U near the surface. As the particles fall through the water carrying the 234 Th with them, they tend to build up at depth, so that the 234 Th activity is noticeably higher where the particles are more abundant. Most of the particles in the upper ocean are biological in origin. This isotope distribution has led people to use 234 Th as a means of modeling the "rain rate" of organic debris through the upper ocean.
The 230 Th daughter has a long half-life (75,300 years); it is also the chemical element thorium, and sticks to particles falling through the ocean, whereas its parent 234 U stays dissolved in sea water. The long half-life al-lows excess 230 Th to build up in sea-floor sediment, with very high values at the surface and decreasing amounts in the older sediment at depth. The change with depth results from the decay of 230 Th over the thousands of years required for a few centimeters of marine sediment to accumulate. The change in 230 Th with depth in the sediment, plus knowledge of the 230 Th half-life, makes it possible to use the information to calculate the deposition rate of sediments on the seafloor.
Artificial Nuclides.
The artificial nuclides in the ocean have originated as global atmospheric fallout resulting from nuclear weapons tests, or from point sources derived from nuclear reactor wastes entering the ocean, or from nuclear accidents. * The atmospheric fallout has a well-known history. Atmospheric weapons tests began in the early 1950s, and continued until 1962 when the atmospheric nuclear test ban treaty was signed by most of the countries that had been conducting the tests. The treaty was prompted by the alarming spread of the radioactive contaminants into the environment and even into the human food supply. 90 Sr was easily detectable in milk, for example. After the test ban, the levels of fallout nuclides decreased abruptly.
One of the products of nuclear weapons testing was tritium. Its distribution in the ocean is like a telltale ink stain revealing the history of its occurrence on Earth. Tritium entered the ocean at the surface and became involved in the sinking North Atlantic water, part of the thermohaline current system that circulates ocean deep water. As the North Atlantic Deep Water moved downward, so did the tritium. Its concentration at various depths and locations gives a picture of a current system at work.
Among the most notorious of the artificial nuclide contaminations on Earth was the Chernobyl reactor accident, which occurred in 1986. The reactor released a plume of radioactive contaminants that changed direction over a few weeks and contributed measurable radioactivity over Europe, Scandinavia, North America, and Russia. The danger in such contamination results from the very high concentrations found in local areas. Additional sources of nuclear contamination from nuclear waste disposal and spills by the former Soviet Union have added a very large amount of radioactivity to the Arctic Ocean.
SEE ALSO Ocean Biogeochemistry ; Ocean Currents ; Radioactive Chemicals ; Tracers of Ocean-Water Masses.
Martha R. Scott
Bibliography
Broecker, W. S. "Geochemical Tracers and Ocean Circulation," in Evolution of Physical Oceanography, B. A. Warren and C. Wunsch, eds. Cambridge, MA: MIT Press, 1981.
Coale, K. H., and K. W. Bruland. "Oceanic Stratified Euphotic Zone as Elucidated by Th-234: U-238 Disequilibria," Limnol. Oceanogr. 32:189–200, 1985.
Eisenbud, Merril, and Thomas F. Gesell, Environmental Radioactivity from Natural, Industrial, and Military Sources, 4th ed. San Francisco, CA: Morgan Kaufmann Publishers, 1997.
Foyn, L. "Radioactive Wastes," in Steele, John H., Karl K. Turekian, and Steve A.Thorpe, eds. Encyclopedia of Ocean Sciences, San Diego, CA: Academic Press, 2001.
Ivanovich, M., and R. S. Harmon, Uranium-Series Disequilibrium: Applications to Earth, Marine, and Environmental Sciences. Oxford, U.K.: Clarendon Press, 1992.
Internet Resources
Radioactivity in the Natural Environment. Lawrence Berkeley National Laboratory. <http://www.lbl.gov/abc/wallchart/chapters/15/3.html> .
Radioactivity in Nature. Idaho State University, Radiation Information Network. <http://www.physics.isu.edu/radinf/natural.htm> .
* See "Radioactive Chemicals" for a photograph related to the Chernobyl accident.